MONJUVI is targeted immunotherapy that effectively binds to CD191
- MONJUVI is an Fc-modified monoclonal antibody that binds to the CD19 antigen expressed on the surface of pre-B and mature B lymphocytes and in several B-cell malignancies, including DLBCL
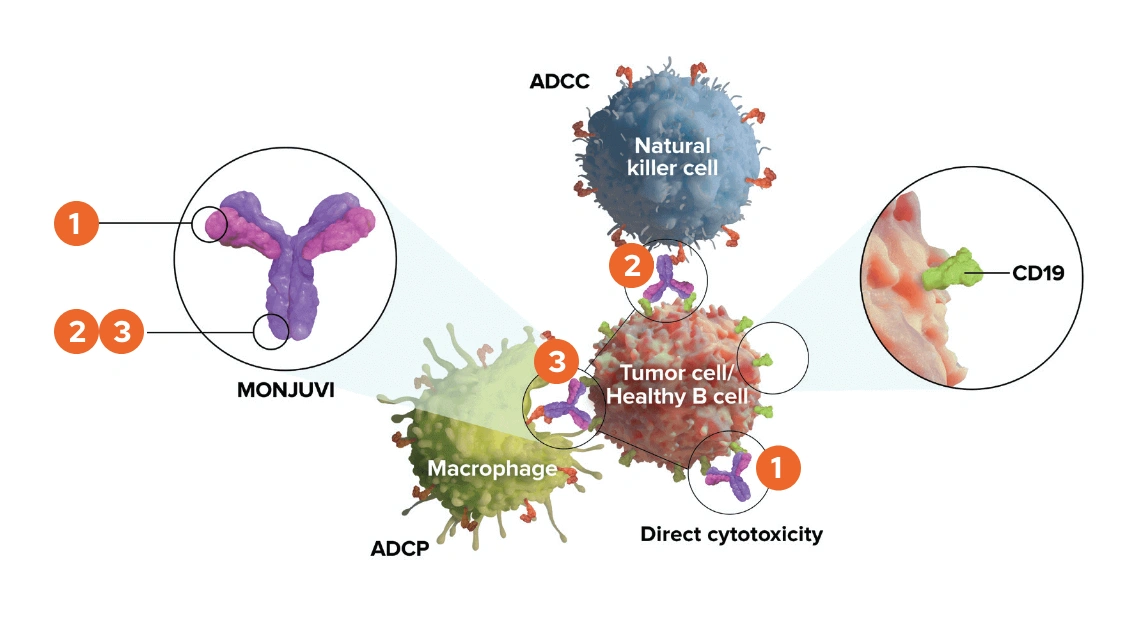
Upon binding to CD19, tafasitamab-cxix mediates B-cell lysis through:
- Apoptosis
- Antibody-dependent cellular cytotoxicity (ADCC)
- Antibody-dependent cellular phagocytosis (ADCP)
Fc=fragment crystallizable.
In studies conducted in vitro in DLBCL tumor cells, tafasitamab-cxix, in combination with lenalidomide, resulted in increased ADCC activity compared to tafasitamab-cxix or lenalidomide alone1
MONJUVI Mechanism of Action Video

MONJUVI (tafasitamab-cxix), in combination with lenalidomide, is indicated for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) not otherwise specified, including DLBCL arising from low-grade lymphoma, and who are not eligible for autologous stem cell transplant (ASCT). This indication is approved under accelerated approval based on overall response rate.
Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial.
Important safety information. Contraindications; None. Warnings and precautions; Infusion related reactions (IRRs); MONJUVI (tafasitamab-cxix) can cause IRRs including fever, chills, rash, flushing, dyspnea, and hypertension. Premedicate patients and monitor frequently during infusion based on the severity of the IRR.
Interrupt or discontinue MONJUVI and institute appropriate medical management. Myelosuppression; MONJUVI can cause serious or severe myelosuppression including neutropenia, lymphopenia, thrombocytopenia, and anemia. Monitor complete blood count (CBCs) before each treatment cycle and throughout treatment. Monitor patients with neutropenia for signs of infection. Consider granulocyte colony-stimulating factor administration. Withhold MONJUVI based on the severity of the adverse reaction.
Refer to the lenalidomide prescribing information for dosage modifications. Infections; Fatal and serious infections, including opportunistic infections, occurred in patients during treatment with MONJUVI and following the last dose. In L-MIND, 73% of the 81 patients with DLBCL who received MONJUVI developed an infection. Grade three or higher infection occurred in 30%. The most frequent infections of any grade were respiratory tract infections 51%, including pneumonias and urinary tract infection (17%). Monitor patients for signs and symptoms of infection and manage infections as appropriate.
Consider infection prophylaxis per institutional guidelines. Consider treatment with subcutaneous or intravenous immunoglobulin (IVIg) as appropriate. Embryo fetal toxicity; Based on its mechanism of action, MONJUVI may cause fetal B-cell depletion when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus and women of reproductive potential to use effective contraception during treatment with MONJUVI and for three months after the last dose.
The combination of MONJUVI with lenalidomide is contraindicated in pregnant women. Refer to the lenalidomide prescribing information on use during pregnancy. Adverse reactions; The most common adverse reactions greater than or equal to 20% in patients with DLBCL were neutropenia 51%, respiratory tract infection 51%, fatigue 38%, anemia 36%, diarrhea 36%, thrombocytopenia 31%, cough 26%, pyrexia 24%, peripheral edema 24%, and decreased appetite 22%.
Please see the Full Prescribing Information available from an Incyte representative or at MONJUVIhcp.com for more information about MONJUVI.
CD19 is a transmembrane glycoprotein that belongs to the immunoglobulin IgG superfamily. It is widely expressed in B-cells, although it can be found in other types of cells. CD19 enhances signaling by the B-cell receptor, which is a transmembrane protein that plays a key role in B-cell development and adaptive immune responses, and recognizes a variety of antigens.
When an antigen engages the B-cell receptor, a cascade of events is triggered inside the cell, starting with phosphorylation of proteins that are dimerized with the B-cell receptor. This recruits several tyrosine kinase proteins that ultimately phosphorylate CD19. This cascade then activates additional kinases and induces cell survival and proliferation.
Diffuse large B-cell lymphoma, or DLBCL, is the most common B-cell non-Hodgkin lymphoma. It is an aggressive lymphoma with diverse clinical presentations and subtypes. CD19 is highly expressed in non-Hodgkin lymphoma, where it induces tumor cell proliferation and survival. This makes CD19 an attractive therapeutic target.
Tafasitamab-cxix is an Fc-modified monoclonal antibody that binds to CD19 antigen expressed on the surface of pre-B and mature B lymphocytes and on several B-cell malignancies, including diffuse large B-cell lymphoma (DLBCL). Tafasitamab is composed of two antigen-binding fragments, or Fabs, specifically targeted against CD19 and a single crystallizable fragment, or Fc. The Fc fragment of an antibody can interact with immune cell surface receptors called Fc receptors.
The modified Fc fragment of tafasitamab was shown to enhance cytotoxic potency. Tafasitamab has three modes of action. These are antibody-dependent cellular cytotoxicity (ATCC) antibody-dependent cellular phagocytosis (ATP) and direct cytotoxicity via apoptosis or programed cell death. In vitro, tafasitamab enhances antibody-dependent cellular cytotoxicity. In vivo, tafasitamab significantly inhibits lymphoma growth. The Fc region of tafasitamab has been engineered to have two amino acid substitutions to increase its affinity to Fc gamma receptors.
Fc gamma receptors can be found on the cell surface of immune cells, such as natural killer cells and delta gamma T-cells, and can bind the Fc portion of immunoglobulins. The two amino acid substitutions in the Fc region of tafasitamab increase antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis in vitro. In addition, the antibody’s affinity to CD19 has been enhanced, and this results in direct tumor cell killing in vitro.
Now let's review the process of ATCC. The first step of ATCC involves antibody binding to the antigen on the cell surface. In the case of tafasitamab, the antibody can bind CD19 on DLBCL cells in vitro. Natural killer cells can recognize and destroy antibody-coated cells. The antibody bound to the cell surface cross-links with Fc receptors found on natural killer cells and gamma delta T-cells.
This crosslinking signals the natural killer cell and gamma delta T-cell to release perforin and granzymes. Perforin and granzymes then cause the destruction of the DLBCL cell.
Another suggested mode of action of tafasitamab is ADCP. The process of ADCP involves binding of an antibody to antigens on tumor cells. Fc receptors on macrophages then recognize the Fc region of the antibody in vitro. The Fc region of tafasitamab binds to human Fc receptors. This initiates Fc dependent phagocytosis where the tumor cell is engulfed by the macrophage.
In vitro, tafasitamab facilitates the antibody-dependent phagocytosis of tumor cells during antibody-dependent phagocytosis. The engulfed tumor cell is internalized by the macrophage and subsequently fuses with membrane-bound sacs called lysosomes that contain digestive enzymes. Upon exposure to these enzymes, the tumor cell dies. The third suggested mechanism by which tafasitamab is thought to kill tumor cells involves direct cytotoxicity via apoptosis.
Note that tafasitamab may also kill healthy cells. In vitro, the enhanced Fc gamma receptor binding of tafasitamab may augment antibody cross-linking and facilitate cell surface CD19 cross-linking, leading to increased caspase-induced apoptosis. Caspases are enzymes involved in the final steps of apoptosis. Apoptosis, or programed cell death, is a process that involves shrinkage of the cell formation of cytoplasm blebs and cell fragmentation.
Important safety information. Contraindications; None.
Warnings and precautions. Infusion-related reactions; MONJUVI (tafasitamab-cxix) can cause infusion-related reactions (IRRs). In L-MIND, infusion-related reactions occurred in 6% of the 81 patients with DLBCL who received MONJUVI. Eighty percent of infusion-related reactions occurred during cycle 1 or 2.
Signs and symptoms included fever, chills, rash, flushing, dyspnea, and hypertension. These reactions were generally managed with temporary interruption of the infusion and/or with supportive medication. Premedicate patients prior to starting MONJUVI infusion. Monitor patients frequently during infusion. Based on the severity of the infusion-related reaction, interrupt or discontinue MONJUVI. Institute appropriate medical management. Myelosuppression; MONJUVI can cause serious or severe myelosuppression including neutropenia, lymphopenia, thrombocytopenia, and anemia.
In L-MIND, among 81 patients with DLBCL who received MONJUVI, Grade three neutropenia was reported in 25%, grade three thrombocytopenia in 12%, and grade three anemia in 7%. Grade four neutropenia was reported in 25% and grade four thrombocytopenia in 6%. Neutropenia led to treatment discontinuation in 3.7% of the patients. Febrile neutropenia occurred in 12%. Monitor complete blood counts (CBCs) before each treatment cycle and throughout treatment.
Monitor patients with neutropenia for signs of infection. Consider granulocyte colony-stimulating factor (G-CSF) administration. Withhold MONJUVI based on the severity of the adverse reaction. Refer to the lenalidomide prescribing information for dosage modifications.
Infections; Fatal and serious infections, including opportunistic infections, occurred in patients during treatment with MONJUVI and following the last dose. In L-MIND, 73% of the 81 patients with DLBCL who received MONJUVI developed an infection.
Grade three or higher infection occurred in 30%. Infection-related deaths occurred in 2.5% of patients, including a case of progressive multifocal leukoencephalopathy (PML). The most frequent grade three or higher infection was pneumonia 7%. The most frequent infections of any grade were respiratory tract infections 51%, including pneumonia and urinary tract infection 17%. Monitor patients for signs and symptoms of infection and manage infections as appropriate.
Consider infection prophylaxis as per institutional guidelines. Consider treatment with subcutaneous or intravenous immunoglobulin IVIg as appropriate. Embryo fetal toxicity. Based on its mechanism of action, MONJUVI may cause fetal B-cell depletion when administered to a pregnant woman. Advise pregnant women of the potential risk to a fetus. Advise women of reproductive potential to use effective contraception during treatment with MONJUVI and for three months after the last dose.
The combination of MONJUVI with lenalidomide is contraindicated in pregnant women because lenalidomide can cause birth defects and death of the unborn child. Refer to the lenalidomide prescribing information on use during pregnancy.
Adverse reactions. Serious adverse reactions occurred in 52% of patients who received MONJUVI. Serious adverse reactions in greater than or equal to 6% of patients included infections 26%, including pneumonia 7%, and febrile neutropenia 6%.
Fatal adverse reactions occurred in 5% of patients who received MONJUVI, including cerebrovascular accident 1.2%, respiratory failure 1.2%, progressive multifocal leukoencephalopathy 1.2%, and sudden death 1.2%. Permanent discontinuation of MONJUVI, or lenalidomide due to an adverse reaction occurred in 25% of patients, and permanent discontinuation of MONJUVI due to an adverse reaction occurred in 15%. The most frequent adverse reactions which resulted in permanent discontinuation of MONJUVI were infections 5%, nervous system disorders 2.5%, and respiratory, thoracic, and mediastinal disorders 2.5%.
Dosage interruptions of MONJUVI or lenalidomide due to an adverse reaction occurred in 69% of patients, and dosage interruption of MONJUVI due to an adverse reaction occurred in 65%. The most frequent adverse reactions, which required a dosage interruption of MONJUVI, were blood and lymphatic system disorders 41% and infections 27%. The most common adverse reactions greater than or equal to 20% were neutropenia, 51%, respiratory tract infection 51%, fatigue 38%, anemia 36%, diarrhea 36%, thrombocytopenia 31%, cough 26%, pyrexia 24%, peripheral edema 24%, and decreased appetite 22%.
Please see the Full Prescribing Information available from an Incyte representative or at MONJUVIhcp.com for more information about MONJUVI.

