L-MIND: an open-label, multicenter, single-arm, Phase 2 study1,2
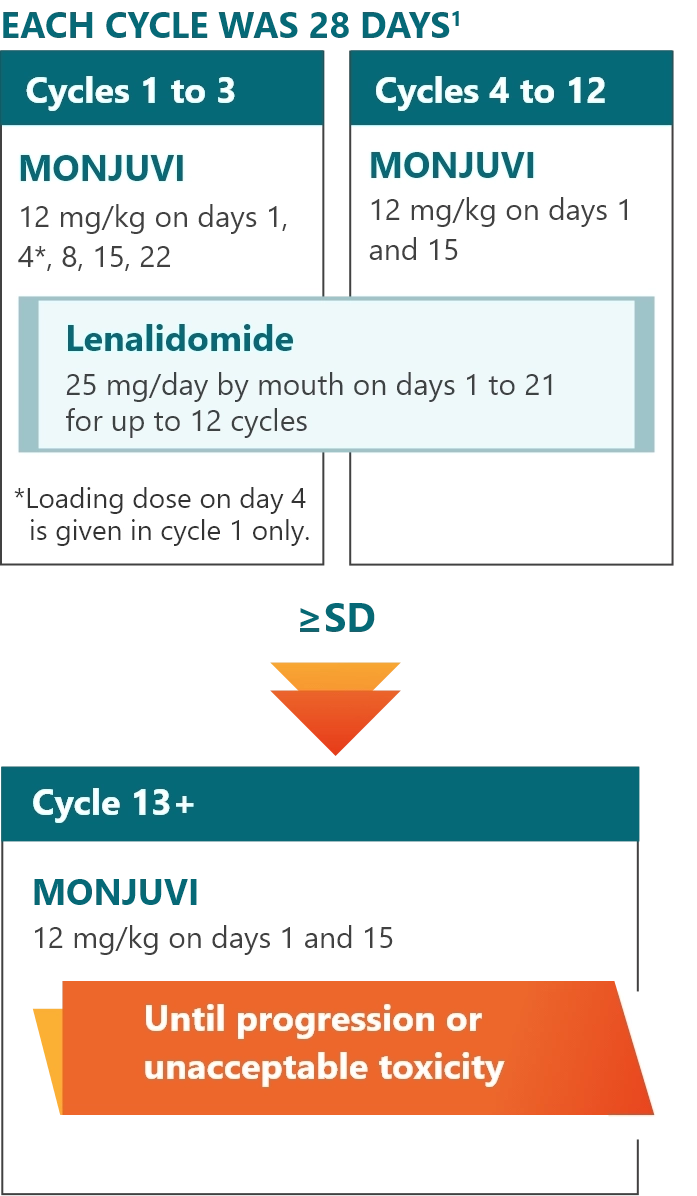
L-MIND study design1
- Efficacy and safety of MONJUVI in combination with lenalidomide followed by MONJUVI monotherapy were evaluated in adults with R/R DLBCL after 1 to 3 prior systemic DLBCL therapies, including a CD20-containing therapy
- Enrolled patients at the time of the trial were not eligible for or refused ASCT
- Efficacy was established in 71 patients with DLBCL, as assessed by an Independent Review Committee using the International Working Group Response Criteria (Cheson 2007)
Primary endpoint2
Best overall response rate (ORR) (CR + PR)
Select secondary endpoint2
Duration of response (DoR)
Administer premedications, including acetaminophen, histamine H1 receptor antagonists, histamine H2 receptor antagonists, and/or glucocorticosteroids, 30 minutes to 2 hours prior to starting MONJUVI infusion to minimize infusion-related reactions.
R/R=relapsed/refractory; DLBCL=diffuse large B-cell lymphoma; ASCT=autologous stem cell transplant; CR=complete response rate; PR=partial response rate; SD=stable disease.
L-MIND examined patients with a broad range of characteristics, including those considered difficult-to-treat1,3-5
Primary refractory patients and those with an IPI score of 3 to 5 were enrolled; in separate analyses, these characteristics of high-risk DLBCL were linked to poor outcomes3-5†
Denotes characteristics of difficult-to-treat patients
Select baseline characteristics (N=71)1,3
| Time between first DLBCL diagnosis and first documented relapse or progression | |
| ≤12 months | 17 (23.9%) |
| >12 months | 53 (74.6%) |
| Unknown | 1 (1.4%) |
| IPI score at screening | |
| 0–2 (low and low-intermediate risk) | 34 (47.9%) |
| 3–5 (intermediate-high and high risk) | 37 (52.1%) |
| Refractory disease | |
| Primary refractory disease‡ | 14 (19.7%) |
| Refractory to last prior therapy | 32 (45%) |
| Refractory to rituximab | 30 (42%) |
| Prior CD20-containing therapy | |
| 100% | |
| Median number of prior therapies | |
| 2 | |
| Prior lines of therapy | |
| 1 | 49% |
| 2 to 4 | 51% |
| Prior ASCT | |
| 9 (13%) | |
| Median age (range) | |
| 71 years (41-86 years) | |
| Race§ | |
| White | 95% |
| Asian | 3% |
| Sex, male | |
| 55% | |
| ECOG performance status | |
| 0 | 26 (36.6%) |
| 1 | 38 (53.5%) |
| 2 | 7 (9.9%) |
| Primary reasons patients were not candidates for ASCT | |
| Age | 47% |
| Refractory to salvage chemotherapy | 27% |
| Comorbidities | 13% |
| Refusal of high-dose chemotherapy/ASCT | 13% |
| Bulky disease|| | |
| 14 (20%) | |
| Elevated LDH | |
| 40 (56.3%) | |
†Based on a 2017 pooled data analysis from patients with refractory DLBCL in 2 observational cohorts and 2 large phase 3 randomized controlled trials (SCHOLAR-1) and a phase 2 trial of patients with stage II to IV newly diagnosed DLBCL and an IPI score of ≥3.
‡Following a protocol amendment, primary refractory DLBCL was defined as no response to or progression/relapse during or within 6 months of frontline therapy. Prior to the amendment, only patients whose disease relapsed within 3 months were defined as primary refractory and excluded. Therefore, patients with disease that relapsed or progressed between 3 and 6 months of frontline therapy were recruited before the protocol amendment.6
§Race was collected in 92% of the 71 patients.1
||Data was collected in 70 patients.3
IPI=International Prognostic Index; ECOG=Eastern Cooperative Oncology Group; LDH=lactate dehydrogenase.

