inMIND: 548 patients with FL were randomized to receive MONJUVI + R2 or placebo + R2 in a Phase 3, double-blind, international, multicenter study1,2
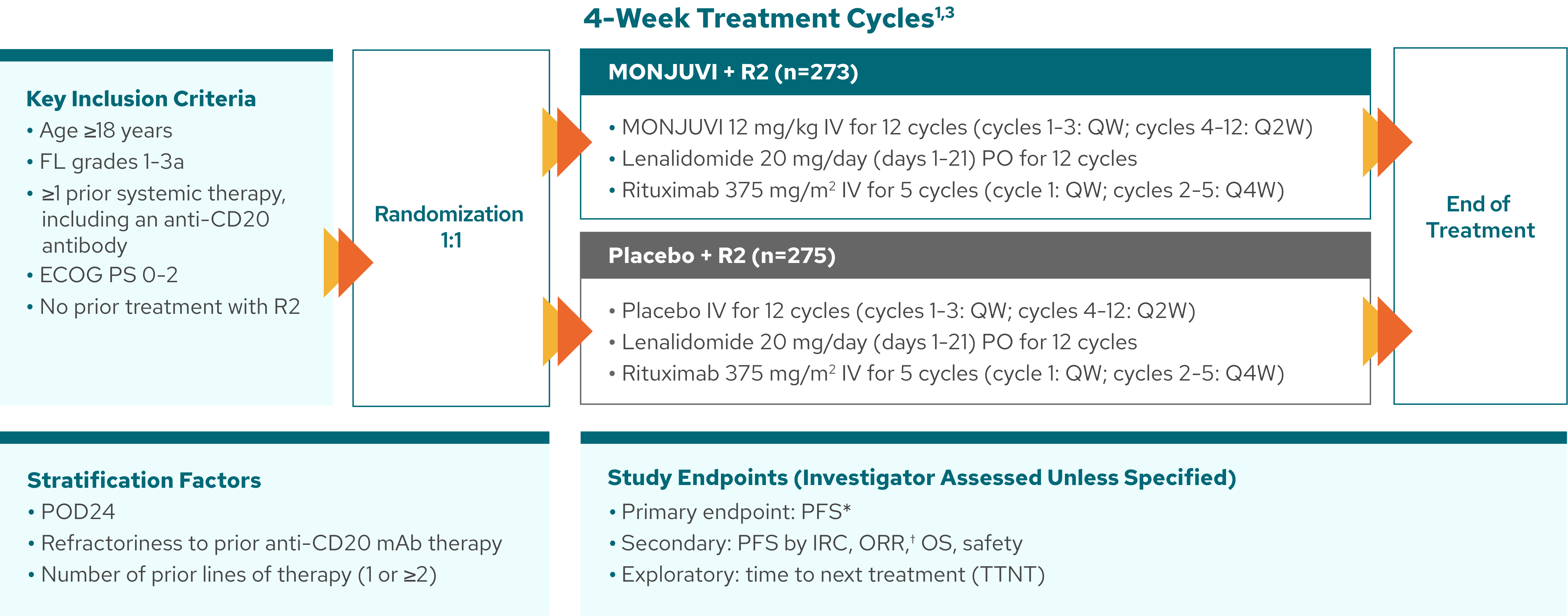
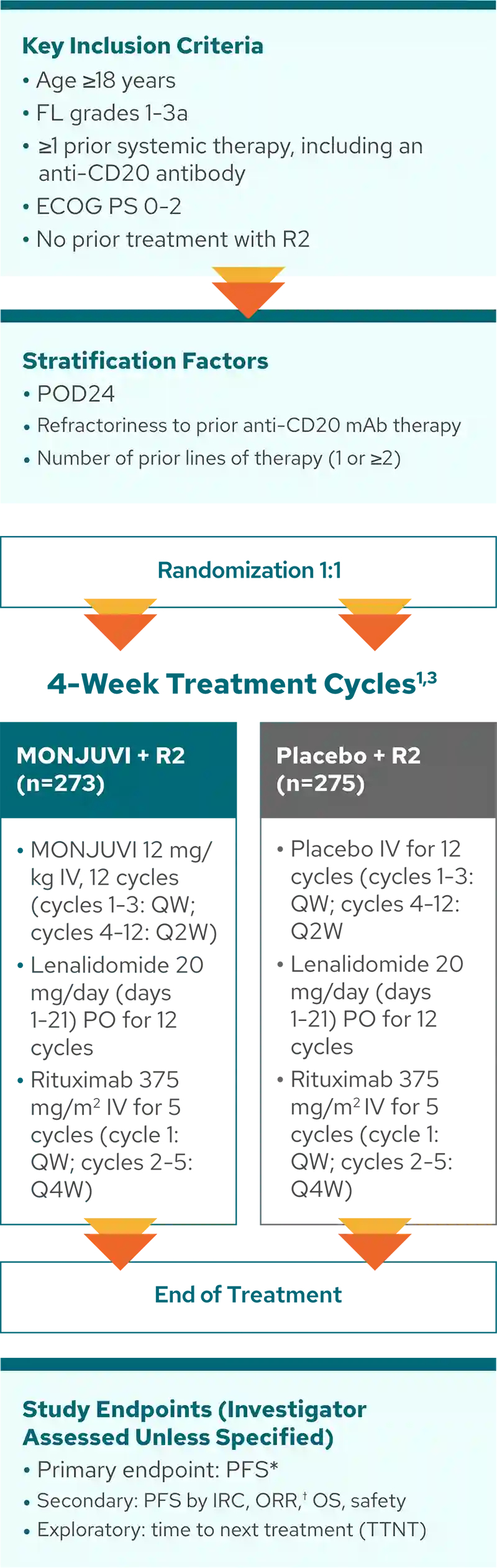
MONJUVI + R2 was administered as a fixed duration
treatment of 12 cycles.1‡
*PFS was defined as the time from randomization to first documented disease progression per Lugano 2014 criteria, or death from any cause, whichever occurs first.4
†Complete response plus partial response.
‡Patients received study treatment for up to 12 cycles in the absence of disease progression or unacceptable toxicity.4
MONJUVI + R2 was studied in a 2L+ FL broad patient population that included tough-to-treat patients1, 5-7
In inMIND, tough-to-treat patients included 83% high tumor burden (met at least 1 GELF criteria), 43% refractory to prior anti-CD20 therapy, and 32% POD24.1,3
Baseline Patient Characteristics1,3,4
| Select Baseline Characteristic | MONJUVI + R2 (n = 273) | Placebo + R2 (n = 275) | ||
|---|---|---|---|---|
| Median age, years (range) | 64 (36, 88) | 64 (31, 85) | ||
| Sex, % | Male | 55 | 54 | |
| Female | 45 | 46 | ||
| Race, %§ | White | 80 | 80 | |
| Asian | 15 | 15 | ||
| Other races | 1 | 2 | ||
| FL grade at trial entry, %‖ | Grade 1 | 22 | 19 | |
| Grade 2 | 52 | 55 | ||
| Grade 3a | 25 | 26 | ||
| At least 1 GELF criteria at baseline, % | 81 | 84 | ||
| POD24, % | 31 | 32 | ||
| Median number of prior lines of therapy (range) | 1 (1, 7) | 1 (1, 10) | ||
| Refractory to most recent prior therapy, % | 41 | 35 | ||
| Refractory to prior anti-CD20 therapy, % | 43 | 42 | ||
| Select Baseline Characteristic | MONJUVI + R2 (n = 273) | |
|---|---|---|
| Median age, years (range) | 64 (36, 88) | |
| Sex, % | Male | 55 |
| Female | 45 | |
| Race, %§ | White | 80 |
| Asian | 15 | |
| Other races | 1 | |
| FL grade at trial entry, %‖ | Grade 1 | 22 |
| Grade 2 | 52 | |
| Grade 3a | 25 | |
| At least 1 GELF criteria at baseline, % | 81 | |
| POD24, % | 31 | |
| Median number of prior lines of therapy (range) | 1 (1, 7) | |
| Refractory to most recent prior therapy, % | 41 | |
| Refractory to prior anti-CD20 therapy, % | 43 | |
| Select Baseline Characteristic | Placebo + R2 (n = 275) | |
|---|---|---|
| Median age, years (range) | 64 (31, 85) | |
| Sex, % | Male | 54 |
| Female | 46 | |
| Race, %§ | White | 80 |
| Asian | 15 | |
| Other races | 2 | |
| FL grade at trial entry, %‖ | Grade 1 | 19 |
| Grade 2 | 55 | |
| Grade 3a | 26 | |
| At least 1 GELF criteria at baseline, % | 84 | |
| POD24, % | 32 | |
| Median number of prior lines of therapy (range) | 1 (1, 10) | |
| Refractory to most recent prior therapy, % | 35 | |
| Refractory to prior anti-CD20 therapy, % | 42 | |
- Refractory lymphoma was defined as achieved less than PR to the last treatment or achieved a CR or PR that lasted <6 months4
- POD24 was defined as progression of disease within 24 months after initial diagnosis1
§Race was not reported in 4% (n=11) of patients in the MONJUVI + R2 arm and 4% (n=10) of patients in the placebo + R2 arm.4
‖ FL grade at trial entry was not reported for 3 patients in the MONJUVI + R2 arm and 1 patient in the placebo + R2 arm.4
