MONJUVI, in combination with lenalidomide, was granted accelerated approval based on the 1-year primary analysis of the L-MIND study.
1-year primary analysis in patients with R/R DLBCL (N=71)1*:
- Best ORR: 55% (n=39; 95% CI: 43%, 67%); CR: 37%; PR: 18%
- Median DoR: 21.7 months (range: 0, 24)†
Review the 1-year primary analysis data here.
The 5-year analysis data from L-MIND have not been submitted to or reviewed by the FDA, and potential inclusion of these data in the final FDA-approved labeling has yet to be determined. This disclaimer applies to all data from the 5-year analysis below.
5-year ORR analysis2*
ORR in patients with R/R DLBCL (N=71)
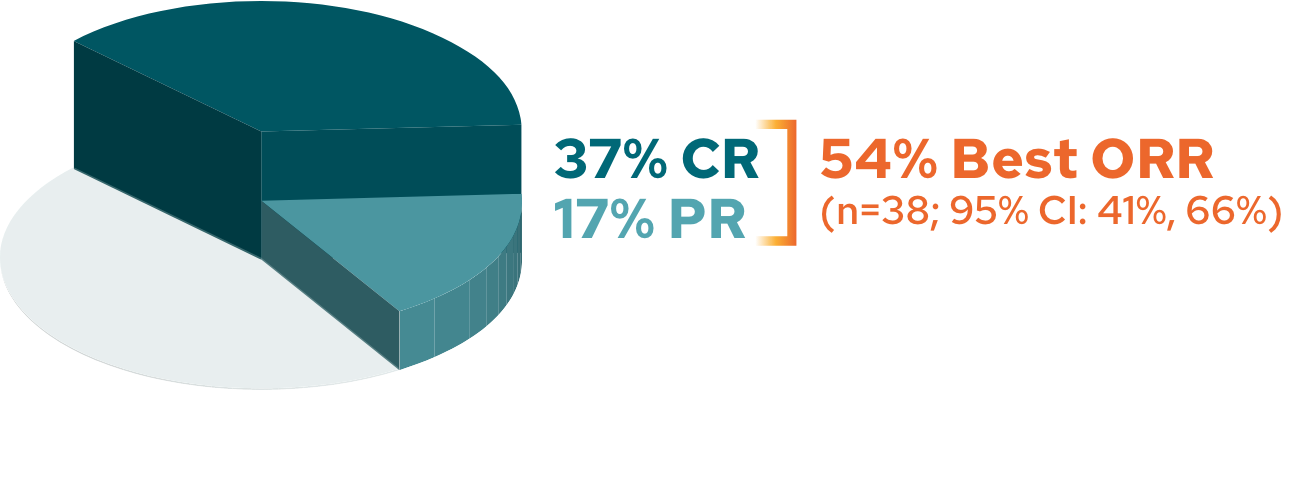

The cutoff date for the 5-year follow-up analysis was November 14, 2022, and occurred after the last patient enrolled had completed 5 years of follow-up3
Response rates in 2L and 3L+2*
Response rates by line of therapy in 5-year analysis
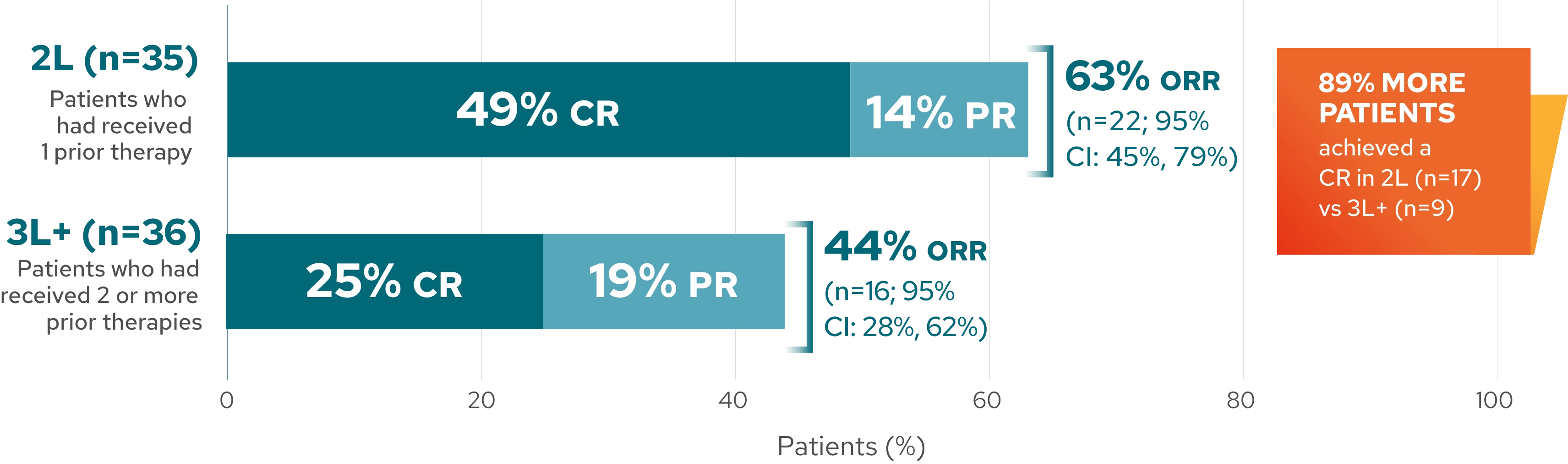
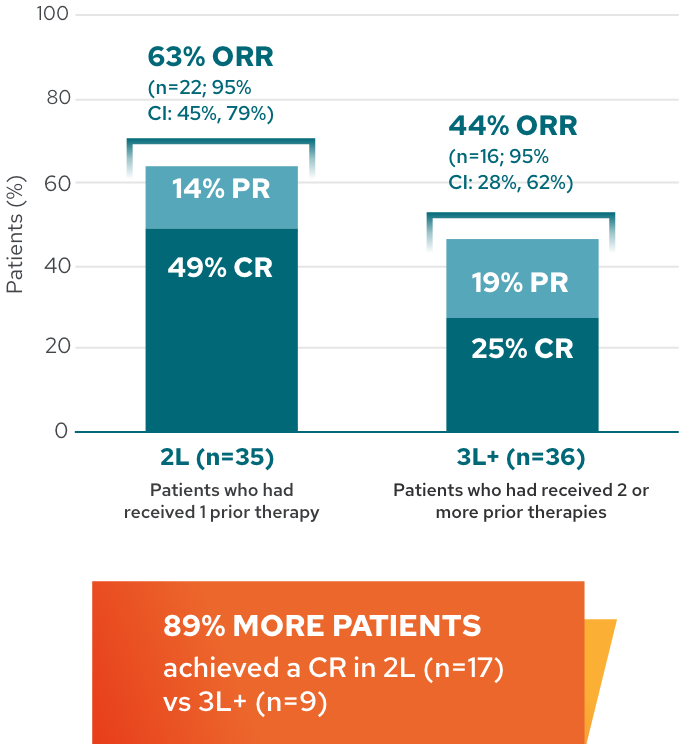
This analysis is exploratory in nature, and L-MIND was not designed or powered to evaluate and compare multiple subgroups. These results should be interpreted with caution given the small sample size, which may lead to estimates that are unstable.
Median DoR in patients with R/R DLBCL2,4
5-year follow-up analysis: median DoR not reached*†
(median follow-up 53.8 months [95% CI: 31.8-58.7])
![L-MIND duration of response Kaplan-Meier graph. A 5-year follow-up analysis in patients with R/R DLCBL (N=71). Median DoR not reached (median follow-up 53.8 months [95% CI: 31.8-58.7]).](/dlbcl/sites/g/files/hssmmz8476/files/2025-11/KM_median-desktop.webp)
![L-MIND duration of response Kaplan-Meier graph. A 5-year follow-up analysis in patients with R/R DLCBL (N=71). Median DoR not reached (median follow-up 53.8 months [95% CI: 31.8-58.7]).](/dlbcl/sites/g/files/hssmmz8476/files/2025-11/KM_median-mobile.webp)
*Assessed by an Independent Review Committee.1
†Kaplan-Meier estimate.1,2
‡DoR rate at 5 years is a Kaplan-Meier estimate and should be interpreted with caution due to the small sample size and the number of censored patients.
The cutoff date for the 5-year follow-up analysis was November 14, 2022, and occurred after the last patient enrolled had completed 5 years of follow-up.3
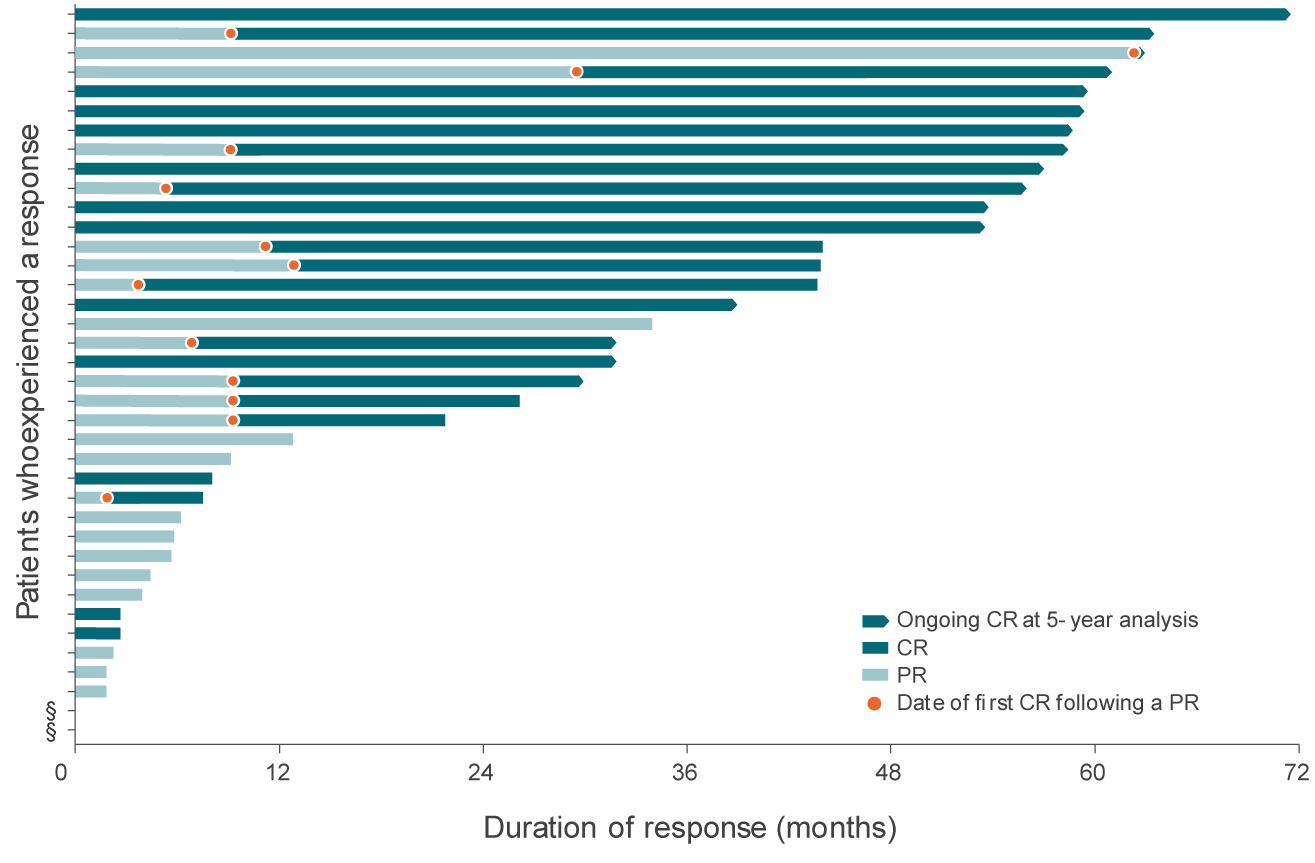
Duration of response by patient4
DoR by patient from time of initial response4


+

Tap to enlarge
- 68% of responding patients (26/38) achieved a best response of CR
- 34% of responders (13/38) converted from a PR to a CR
- 32% of responding patients (12/38) had a response of at least 4 years
This graph and these bullets represent 38 out of the 71 patients in the L-MIND study who experienced a response; 33 patients did not experience a complete or partial response.
For patients whose response has stopped, events may include tumor progression, death, or beginning of a new anti-cancer treatment.
The study concluded on the 5-year analysis cutoff date of November 14, 2022.4
This analysis is exploratory in nature. These results should be interpreted with caution due to single-arm studies not adequately characterizing time-to-event endpoints, and the small sample size, which may lead to estimates that are unstable.
This analysis is exploratory in nature. These results should be interpreted with caution due to single-arm studies not adequately characterizing time-to-event endpoints, and the small sample size, which may lead to estimates that are unstable.
§Two patients started a new anti-cancer treatment immediately after a response was recorded at the end of the treatment assessment.4
The initial assessment of efficacy/disease response was performed and recorded at Cycle 3, Day 1.4
The cutoff date for the 5-year follow-up analysis was November 14, 2022, and occurred after the last patient enrolled had completed 5 years of follow-up.3
L-MIND study design
- L-MIND was an open-label, multicenter, single-arm, Phase 2 study in adult patients with R/R DLBCL who were not eligible for or refused ASCT.1,5
- Efficacy was established based on best ORR (defined as the proportion of complete and partial responders), and DoR, as assessed by an Independent Review Committee using the International Working Group Response Criteria (Cheson 2007).1
See the full study design here.

